Genetic Analysis to Predict the Development of Paget’s Disease
The purpose of the study is to develop genetic markers that can be used to predict the development of Paget’s disease of bone (PDB) in people who have a family history of the disease.
What is the purpose of the study?
The purpose of the study is to develop genetic markers that can be used to predict the development of Paget’s disease of bone (PDB) in people who have a family history of the disease.It is known that people with a family history of PDB are at a 7-times increased risk of developing the disease themselves in later life compared with the general population. We are therefore inviting people who have a parent or sibling with PDB to take part in this study. The study will involve screening for the presence of early PDB using a test called a radionuclide bone scan which is a highly sensitive way of picking up early PDB. We will also conduct an analysis of a blood samples for genetic markers and biochemical markers as well as the profile of bacteria in the mouth and gut (the microbiome). By correlating these markers with the presence or absence of PDB on the bone scan we hope to be able to develop a test which can be used in the future to assess the likelihood of developing PDB in people with a family history of the condition.


Why have I been invited to take part?
You have been asked to take part because you have a parent or sibling with PDB, or you are a spouse of an individual with a family history of PDB.

Do I have to take part?
No, it is up to you to decide whether or not to take part. If you do decide to take part you will be given this information sheet to keep and be asked to sign the consent form which is at the end of this information sheet. If you decide to take part but later change your mind, you would be free to withdraw from the study at any time and without giving a reason. Deciding not to take part or withdrawing from the study will not affect the healthcare that you receive, or your legal rights.
What will happen if I take part?
Baseline Visit
To begin with, your consent will be obtained by one of the doctors or nurses in the study team.
As a part of the visit, you will a radionuclide bone scan unless you have already had one in the previous two years.
This is a highly sensitive way of picking up the presence of PDB long before other signs or symptoms are present.
In addition to the bone scan we will ask you to provide a blood sample (about 36ml which is the equivalent of about seven teaspoons) and to provide a saliva sample and a stool sample.
The saliva and stool samples can be collected at home using special packs and posted free of charge to the study centre.
We will also ask you to complete some questionnaires to assess your diet, physical activity, any pain you may be experiencing and your quality of life.
Overall the study visit will take about 3 hours (including the time taken for the scan).
Control participants will only undertake the Baseline visit and there will not be a bone scan for them.

Remote Visits
After the first visit we will keep in contact with you after 15 months and again after 45 months by phone, email or text message (depending on your preferred method of contact) to ask if there have been any changes in your general health and to check if you have received any medications that affect the bones.These contacts are estimated to take about 15 minutes of your time.
30 Month Visit
You will be offered another clinic visit after 30 months (which will mark the half-way stage of GAPDPD).
At this point we will repeat the blood tests (20ml, or four teaspoons) and ask you to complete questionnaires about any pain you may be having and questionnaires about quality of life.
This visit will take about 1 hour of your time.
It will usually be performed by visiting the study centre, but it may be possible for you to have the samples taken by your GP.


End Of Study
The final visit of GAPDPD will performed about 60 months (5 years). At this point we will repeat the same tests as you had at the first visit including the radionuclide bone scan to see if any signs of PDB have developed.
This visit is expected to take about 3 hours as for the baseline visit.
Bone Scan
Bloods
Stool and Saliva
Questionnaires
Frequently Asked Questions


Will any genetic research be performed in the study?
Yes. We are planning to perform genetic studies to try and develop a test that can predict the presence of PDB. We will use your blood samples to look for changes in your genetic material (DNA/RNA) that may be linked to Paget’s disease. We will use your blood samples to study whether genes that might play a role in Paget’s disease are turned on or off. This may include the use of techniques called whole genome analysis or exome analysis in which we can comprehensively search for genetic variants that predispose to the disease. We will give you the opportunity of being given the results of your genetic profile if you wish and an estimate of your individual risk of developing PDB. The blood samples will also be used to determine if there are any changes in bone metabolism by analysing what is termed “biochemical markers” of bone turnover. This can give an insight into the rate at which the skeleton is being repaired and renewed. Your stool and saliva samples will be used to study what kinds of bacteria are present in your gut. This will allow researchers to try and determine if certain kinds of gut bacteria are associated with the development of Paget’s disease.

What does treatment for Paget’s disease involve?
There are several options but the most frequently used is a drug called zoledronic acid which is given by a drip or infusion. Other treatments are also available in the form of a course of tablets. If it looks like you might need treatment for Paget’s disease a member of the study team will discuss all of the treatment options with you and the potential side effects.

What are the possible benefits of taking part?
A potential benefit of you taking part is that you will be kept under surveillance for any signs or symptoms of Paget’s disease. This could be helpful since we would be able to offer you treatment for the disease if appropriate. The study will also enable us to develop a test for PDB which could help future generations of people with a family history of the disease.

What are the possible disadvantages of taking part?
If you are a participant with a family history of PDB, We will be asking you to have a bone scan at the beginning and end of the GAPDPD study. An x-ray may also be performed if the scan shows possible signs of Paget’s disease. Both procedures involve exposure to ionising radiation which can cause cell damage. We are all exposed to low amounts of ionising radiation as part of normal life due to the presence of naturally-occurring radioactive materials found in soil, water and air and exposure to cosmic rays produced by the sun. This is called background radiation. Taking part in this study will result in additional exposure to ionisation radiation due to the bone scan and x-ray. However, the total dose of radiation you will receive if you take part in this study is low and equivalent to about three years background radiation in the UK. Cells that are exposed to ionising radiation may, after many years or decades, turn cancerous. We are all at risk of developing cancer during our lifetime. The normal risk is that this will happen to about 50% of people at some point in their life. Taking part in this study will add only a very small chance (approximately 0.03%) of this happening to you.
You might experience some mild discomfort as the blood sample is taken.
There is a very small possibility that the blood tests or bone scan performed during the study might pick up abnormalities unrelated to Paget’s disease. If this happens and the study team think the abnormality might need treatment we will inform you and your general practitioner about the results, so that appropriate action can be taken.
To our knowledge, participation in the study should not affect your medical insurance or travel insurance. If you are found to develop Paget’s disease during the study then you should of course mention that to your insurance company.

What if the tests that I have during the study pick up something unexpected?
If the blood tests and scans performed as the result of the study detect an abnormality, which is unexpected and which we think might need treatment, we will inform you and your general practitioner so that appropriate action can be taken. This will only be done if the abnormality is thought to require treatment and is considered to represents a threat to your health and well-being.

Will I need to have any treatment for Paget’s disease of bone during the study?
No, not necessarily. If you develop symptoms that we think are caused by Paget’s disease, we will communicate this information to your GP and suggest that you are offered you one of the standard treatments for the disease. This is what would happen normally even if you weren’t taking part in this study.

Is there anything I need to do or avoid?
Not particularly. There isn’t anything special that you need to do or avoid if you take part in the study. The only thing to mention to the study team is if you are a woman and you decide you might want to get pregnant during the study. There isn’t any issue with this but it’s important that you mention to a member of the study team if you think you are pregnant to ensure that you don’t have a bone scan or x-rays. If you think there is a possibility that you might be pregnant before having the bone scan a member of the study team will ask you to provide a urine sample so that you can be tested for possible pregnancy before going ahead with the scan. If you are breastfeeding, then you can still take part but the bone scan would need to be delayed until after you had stopped breastfeeding.
The radioactive substance used in the bone scan takes a couple of days to disappear from your body. Because of this, you will be asked to avoid young children and pregnant women for 48 hours after the scan and to avoid giving blood, stool and urine samples and avoid undergoing surgery (including dentistry) for the same amount of time.

What will happen if I don’t want to carry on with the study?
You are free to withdraw from the study at any time. If you decide to withdraw you don’t have to give us a reason why, and your decision to withdraw will not affect the standard of medical care that you receive. If you choose to withdraw from the study you will be given options. The first would be for you to stop attending study visits but still to attend for the end of study visit and be followed up remotely. The second would be for you to withdraw from all further study visits but agree to be followed up remotely. The third would be complete withdrawal from all study activities. If you withdraw from the study, we will keep the information about you that we have already obtained. If we have not yet analysed your study samples, you can request that we destroy these. If for any reason you develop an illness that means you lose the ability to make decisions and to continue follow up visits during the study, we will keep the samples and data we hold from you until that time.

What if there are any problems?
If you have a concern about any aspect of this study you will be able to contact your local principal investigator by telephone or by email and they will do their best to answer your questions. If something goes wrong and you are harmed during the research and this is due to someone’s negligence then you may have grounds for a legal action for compensation but you may have to pay your legal costs.
Confidentiality

Will my taking part be kept confidential?
Yes. All the information we collect during the course of the research will be kept confidential and there are strict laws which safeguard your privacy at every stage. The data we collect about you will be kept secure by storage on computer systems at the University of Edinburgh. We will ask you to consent to us holding personal data and your NHS or CHI number (or equivalent number in other health care systems) so that members of the study team can contact you about the study and feedback results. We will also seek your permission to access your medical records as part of the GAPDPD study. Access to your data will be restricted to members of the study team on secure computers which will be protected by usernames and passwords. In order to maximise security, we will ensure that any personal data we hold about you is in a different file to other study data which will contain the results of your blood tests, scans and questionnaires. Any paper records we hold about you such as consent forms will be stored in locked cabinets within secure buildings to which only members of the study team have access.
We may share data from the study with other doctors and scientists who are conducting research on Paget’s disease and other diseases in the UK, Europe and other countries. If we do this, we will only share what’s called “anonymised” data. This means that any data that is shared would not include any personal details and could not be used to identify with you. The principal investigator of the study, Professor Stuart H Ralston will be responsible for ensuring that your data is held securely. In order to monitor and audit the study we will ask your consent for responsible representatives from the sponsor(s) and NHS Institution(s) to access your medical records and data collected during the study, where it is relevant to you taking part in this research. The Sponsor(s) are responsible for overall management of the study and providing insurance and indemnity.
We will inform your GP that you are taking part in the study provided that you consent to us doing so.
Important Study Information


Who is organising and funding the research?
This study has been organised by the University of Edinburgh and is jointly sponsored by the University of Edinburgh and NHS Lothian. The study is being funded by the European Commission and the University of Edinburgh. The doctors and nurses involved in the study are not receiving any personal payments for the study but the costs involved of the blood tests, bone scans and x-rays are being reimbursed

Who has reviewed the study?
The study proposal has been reviewed by the European Commission and its medical and scientific advisors and by members of the Paget’s Association, a patient support organisation. The study has also been evaluated and approved by the East of Scotland Research Ethics Committee which has responsibility for scrutinising all proposals for medical research on humans. It is a requirement that your records in this research, together with any relevant medical records, be made available for scrutiny by monitors from the University of Edinburgh and NHS Lothian, whose role is to check that research is properly conducted and the interests of those taking part are adequately protected.
Site Locations
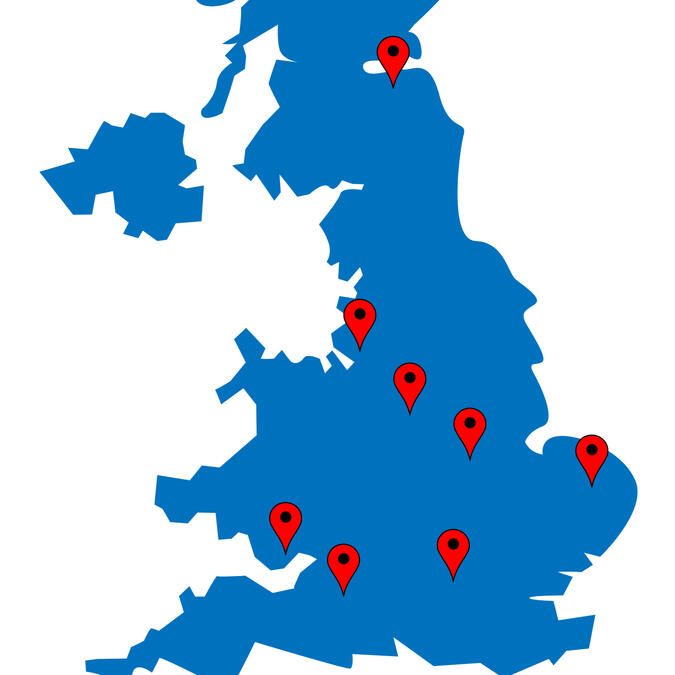
Where can I take part?
We are pleased to announce that our clinical trial is now actively recruiting participants at multiple sites across the United Kingdom. Currently open recruitment centers include:
-Western General Hospital in Edinburgh;
-Cardiff and Vale University Health Board at University Hospital Llandough;
- Guy's and St Thomas' Hospital NHS Trust in London;
- Midlands Partnership NHS Foundation Trust at Haywood Hospital in Stoke-on-Trent;
-Royal United Hospital Foundation Trust in Bath;
-University Hospital Aintree in Liverpool;
-University Hospitals of Leicester NHS Trust at Leicester General Hospital;
-Norfolk and Norwich University Hospitals NHS Foundation Trust at the Clinical Research Facility in Norwich.
If you're interested in participating and are located near any of these sites, please contact us, and we pass your details on to the respective centre who will provide more information on eligibility and enrollment.
Contact Us
Thank you for taking the time to review our study
If you would like more information on the GAPDPD trial, please contact us either by phone 0131 651 8741 or email [email protected]
